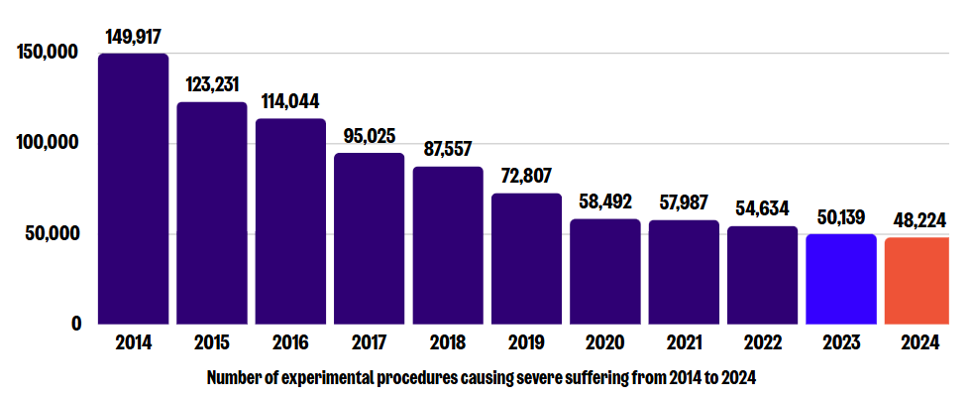
A decade has passed since the recording of actual severity was first reported. In this post we’ll take a look at the latest figures on severe procedures from last year, and the initial data from back in 2014.
The most recent statistics on animal research in the UK, for 2024, were released on 23 October. We welcomed yet another decrease in the number of experimental procedures causing severe suffering, which fell to 48,224 in 2024 (down from 50,139 in 2023*, and 149,917 in 2014* – an overall decrease since then of 68%). This continued decline represents further progress towards our goal of ending severe suffering in animal research.
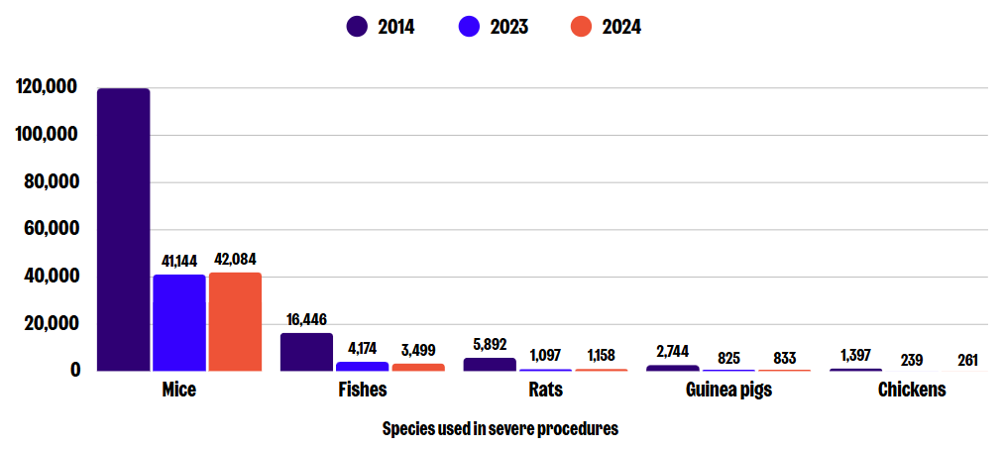
Species
Mice are most commonly used in severe procedures, followed by fishes (including zebrafish), and rats. Other species that have experienced severe suffering include guinea pigs, hamsters, rabbits, cattle, pigs, sheep, dogs, monkeys, frogs, and chickens.
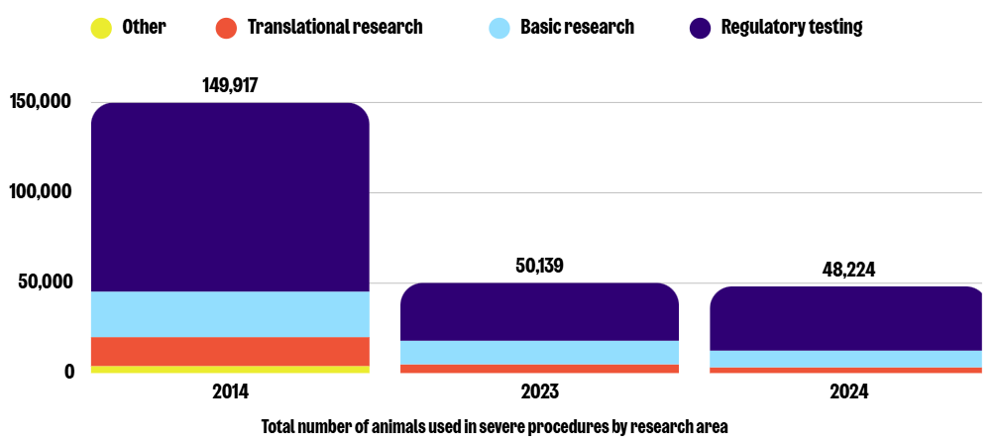
Purpose of procedures
Similarly to 2023, the three most common purposes of severe procedures in 2024 were regulatory testing (74%), basic research (19%), and translational research (7%). While severe procedures in regulatory testing increased by 11% in 2024, animal use in basic and translational research decreased by 29% and 32%, respectively. This represents a decrease of 66%, 63%, and 78% in these same areas compared to 2014.
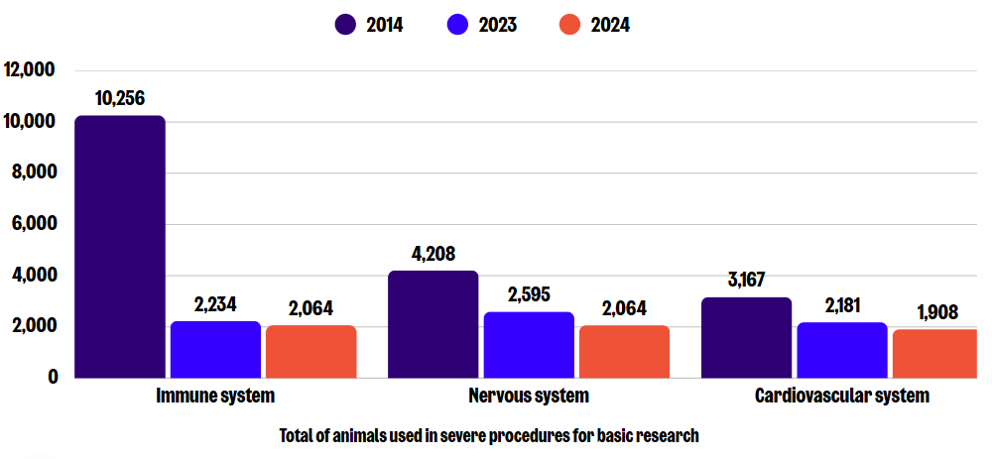
Sub-categories of severe use: Basic research
In 2024, a total of 9,243 severe procedures were carried out for basic research, which is a 29% decrease compared to 2023, and an overall reduction of 63% since 2014. The greatest numbers in this sub-category were reported for research on the immune, nervous, and cardiovascular blood and lymphatic systems. Notably, the number of procedures conducted in each of these areas slightly decreased from those conducted in 2023.
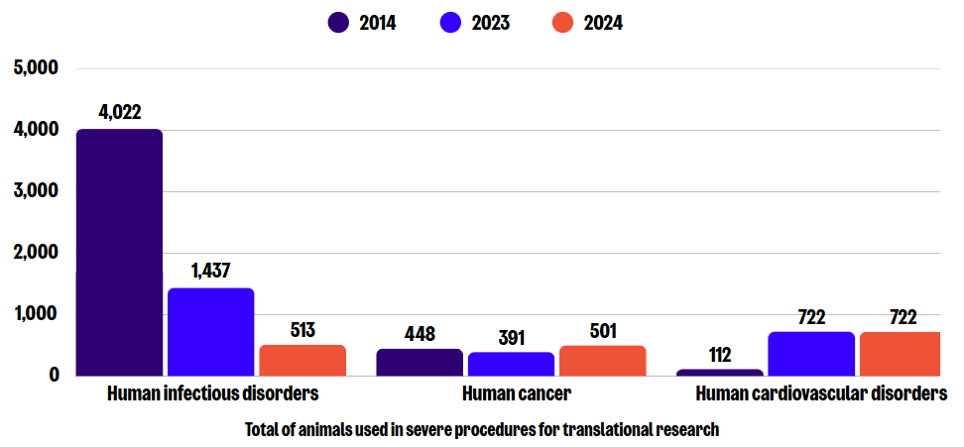
Sub-categories of severe use: Translational research
In 2024, a total of 3,467 severe procedures were conducted for translational research, which is a 32% decrease compared to 2023, and an overall reduction of 78% since 2014. In this sub-category, human cardiovascular and infectious disorders, and human cancer were the leading causes of severe animal use. Compared to 2023, procedures for human infectious disorders decreased by 64%, while those for human cancer increased by 28%.
Sub-categories of severe use: Regulatory use
In 2024, there were a total of 35,511 severe procedures for regulatory use, which is an 11% increase compared to 2023, but in the context of an overall reduction of 66% since 2014. The greatest numbers in this sub-category were reported for batch potency testing, acute and sub-toxicity testing methods (LD50 and LC50), and ecotoxicity (chronic toxicity) testing. Compared to 2023, the number of severe procedures increased by 14%, 8%, and 11%, respectively.
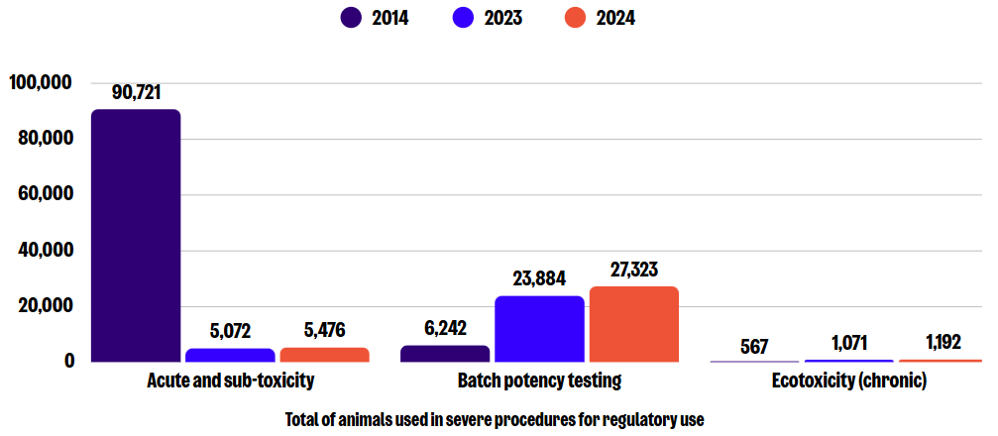
It is likely that the significant drop in severe procedures for the immune system and human infectious disorders categories is because some regulatory tests and production methods have been replaced with non-animal alternatives. For example, the National Institute for Biological Standards and Controls (NIBSC), the UK’s National Control Laboratory, has reduced its total animal use by over 76% from 2014.
Unfortunately, this progress is partly undermined by the increase in severe procedures for regulatory use. One of the most prominent examples of batch potency testing is the mouse LD50 test for botulinum toxin (Bt). The use of Bt for cosmetic purposes has greatly increased demand, with 7.8 million of botox treatments applied in 2024 worldwide, according to the International Society of Aesthetic Plastic Surgery. Although testing cosmetics on animals is banned in the UK, there is a legal loophole in place because Bt also has legitimate medical uses, and companies also do not share validated non-animal tests.
Besides the replacement of animals, the refinement of procedures has also contributed to the significant reduction in these figures. For example, in models for severe diseases like sepsis, death was traditionally used as the scientific endpoint. However, major refinements such as using advanced monitoring techniques and improving welfare assessment have played a pivotal role in establishing early humane endpoints and, therefore, reducing severity from ‘severe’ down to ‘moderate’. Real-life examples of the application of refinements to successfully reduce or avoid severe suffering can be found here.
To accelerate this progress, we have created the Roadmap to reducing suffering, which is a practical exercise designed to help you focus on procedures in your institution that could cause severe suffering, identify contributing factors and find ways of avoiding or refining these.
These are positive times with respect to the development, validation and uptake of non-animal methods to replace procedures at all levels of severity. The recent publication of the UK government’s strategy to phase out the use of animals in research and testing is a significant step and a clear sign of progress toward this goal, but substantial work remains to be done. For example, the continuing requirement for animal use to fulfil regulatory requirements highlights one of the main challenges, but the inclusion of ending regulatory testing in some important areas within the recent phase-out strategy is a promising first step.
Our primary goal is the complete replacement of all animal experiments with humane alternatives. We believe that every individual animal, of every species, is important, including mice, rats and fishes. We are committed to replacing animals wherever possible, reducing the number of animals used, minimising their suffering, and improving their welfare for as long as animal research continues.
Read the full statistics on animal use here.
Read the UK government’s strategy to phase out animals here.
*Data for 2014 and 2023 used in this post are corrected and revised. It is standard practice across all Home Office statistical releases to incorporate revisions to previous years’ data in the latest release. For more information, please visit the User guide to annual statistics of scientific procedures on living animals.
