Latest

A decade of data on severe procedures in the UK
A decade has passed since the recording of actual severity was first reported. In this post we’ll take a look at the latest figures on severe procedures from last year, and the initial data from back in 2014.
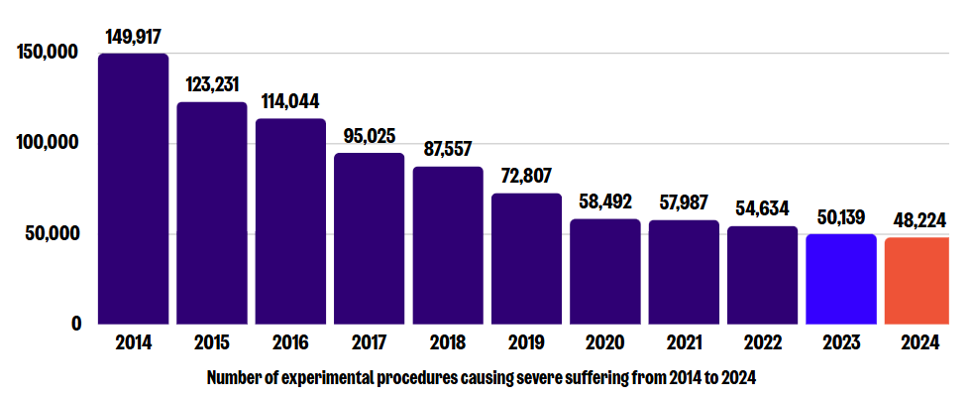
The most recent statistics on animal research in the UK, for 2024, were released on 23 October. We welcomed yet another decrease in the number of experimental procedures causing severe suffering, which fell to 48,224 in 2024 (down from 50,139 in 2023*, and 149,917 in 2014* – an overall decrease since then of 68%). This continued decline represents further progress towards our goal of ending severe suffering in animal research.
Species
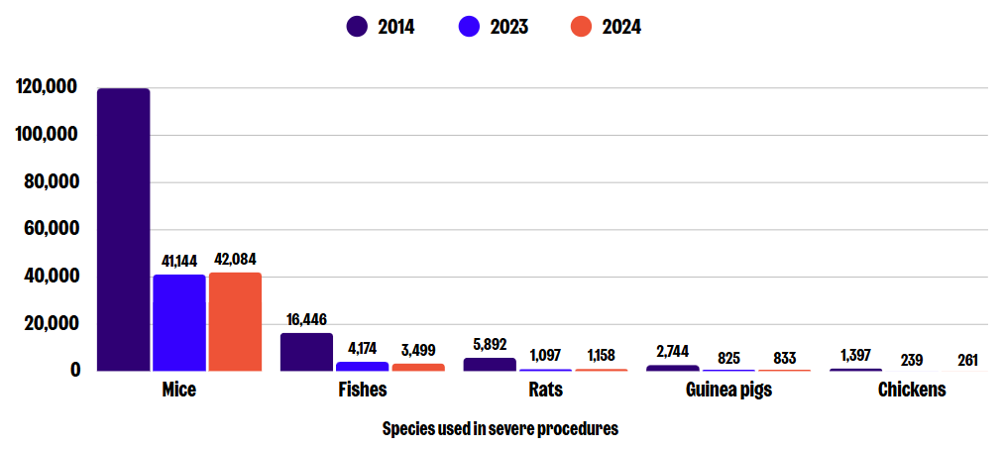
Mice are most commonly used in severe procedures, followed by fishes (including zebrafish), and rats. Other species that have experienced severe suffering include guinea pigs, hamsters, rabbits, cattle, pigs, sheep, dogs, monkeys, frogs, and chickens.
Purpose of procedures
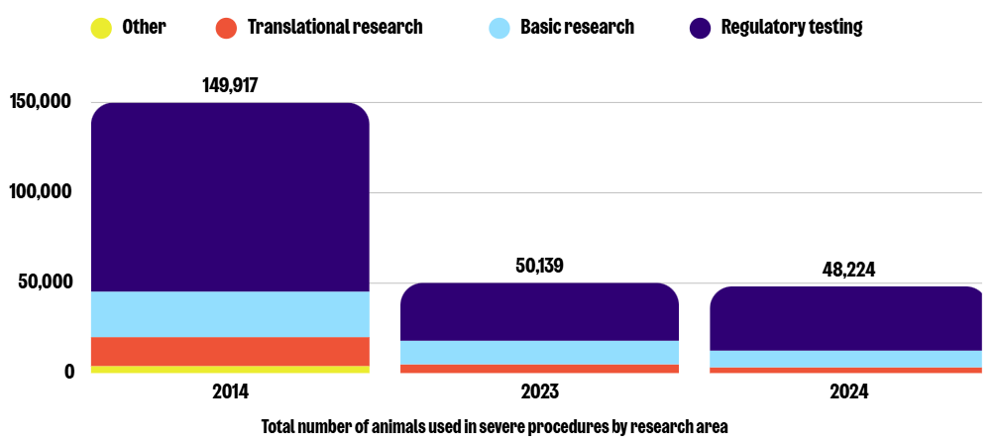
Similarly to 2023, the three most common purposes of severe procedures in 2024 were regulatory testing (74%), basic research (19%), and translational research (7%). While severe procedures in regulatory testing increased by 11% in 2024, animal use in basic and translational research decreased by 29% and 32%, respectively. This represents a decrease of 66%, 63%, and 78% in these same areas compared to 2014.
Sub-categories of severe use: Basic research
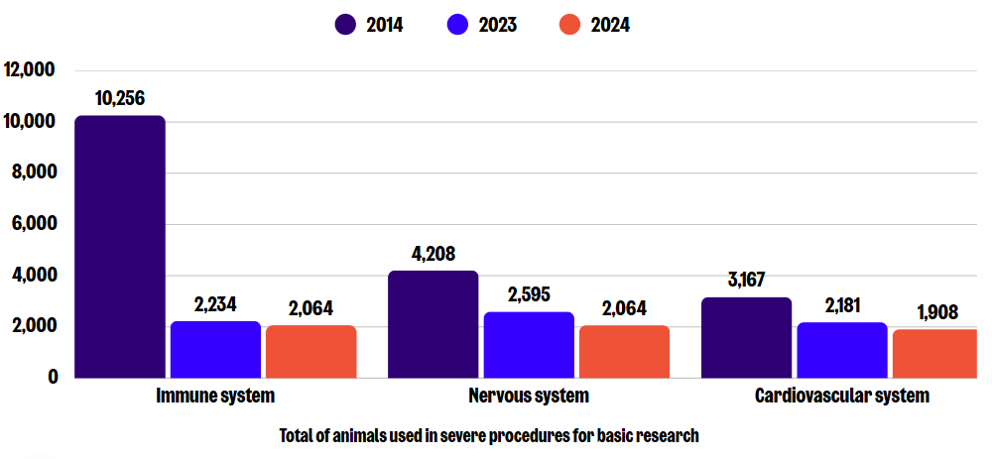
In 2024, a total of 9,243 severe procedures were carried out for basic research, which is a 29% decrease compared to 2023, and an overall reduction of 63% since 2014. The greatest numbers in this sub-category were reported for research on the immune, nervous, and cardiovascular blood and lymphatic systems. Notably, the number of procedures conducted in each of these areas slightly decreased from those conducted in 2023.
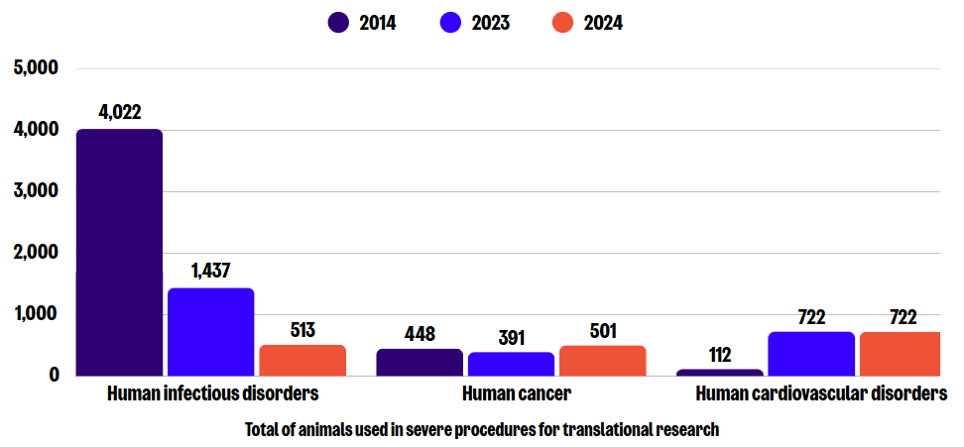
Sub-categories of severe use: Translational research
In 2024, a total of 3,467 severe procedures were conducted for translational research, which is a 32% decrease compared to 2023, and an overall reduction of 78% since 2014. In this sub-category, human cardiovascular and infectious disorders, and human cancer were the leading causes of severe animal use. Compared to 2023, procedures for human infectious disorders decreased by 64%, while those for human cancer increased by 28%.
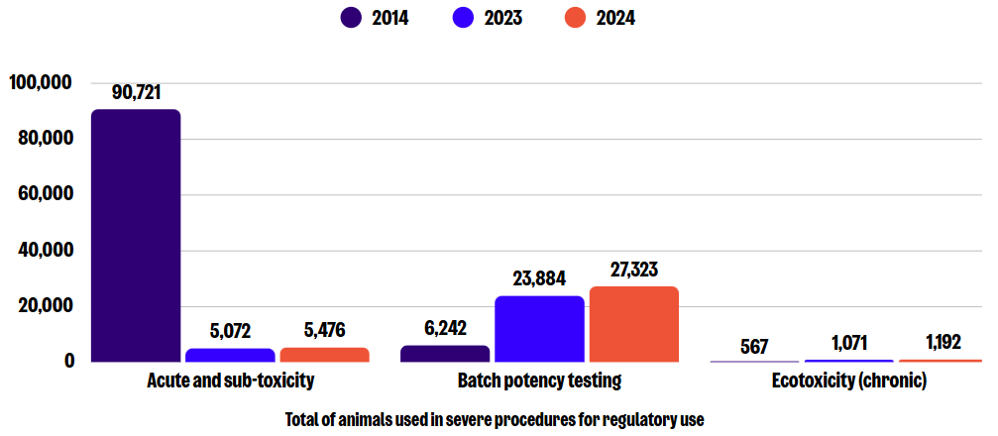
Sub-categories of severe use: Regulatory use
In 2024, there were a total of 35,511 severe procedures for regulatory use, which is an 11% increase compared to 2023, but in the context of an overall reduction of 66% since 2014. The greatest numbers in this sub-category were reported for batch potency testing, acute and sub-toxicity testing methods (LD50 and LC50), and ecotoxicity (chronic toxicity) testing. Compared to 2023, the number of severe procedures increased by 14%, 8%, and 11%, respectively.
It is likely that the significant drop in severe procedures for the immune system and human infectious disorders categories is because some regulatory tests and production methods have been replaced with non-animal alternatives. For example, the National Institute for Biological Standards and Controls (NIBSC), the UK’s National Control Laboratory, has reduced its total animal use by over 76% from 2014.
Unfortunately, this progress is partly undermined by the increase in severe procedures for regulatory use. One of the most prominent examples of batch potency testing is the mouse LD50 test for botulinum toxin (Bt). The use of Bt for cosmetic purposes has greatly increased demand, with 7.8 million of botox treatments applied in 2024 worldwide, according to the International Society of Aesthetic Plastic Surgery. Although testing cosmetics on animals is banned in the UK, there is a legal loophole in place because Bt also has legitimate medical uses, and companies also do not share validated non-animal tests.
Besides the replacement of animals, the refinement of procedures has also contributed to the significant reduction in these figures. For example, in models for severe diseases like sepsis, death was traditionally used as the scientific endpoint. However, major refinements such as using advanced monitoring techniques and improving welfare assessment have played a pivotal role in establishing early humane endpoints and, therefore, reducing severity from ‘severe’ down to ‘moderate’. Real-life examples of the application of refinements to successfully reduce or avoid severe suffering can be found here.
To accelerate this progress, we have created the Roadmap to reducing suffering, which is a practical exercise designed to help you focus on procedures in your institution that could cause severe suffering, identify contributing factors and find ways of avoiding or refining these.
These are positive times with respect to the development, validation and uptake of non-animal methods to replace procedures at all levels of severity. The recent publication of the UK government’s strategy to phase out the use of animals in research and testing is a significant step and a clear sign of progress toward this goal, but substantial work remains to be done. For example, the continuing requirement for animal use to fulfil regulatory requirements highlights one of the main challenges, but the inclusion of ending regulatory testing in some important areas within the recent phase-out strategy is a promising first step.
Our primary goal is the complete replacement of all animal experiments with humane alternatives. We believe that every individual animal, of every species, is important, including mice, rats and fishes. We are committed to replacing animals wherever possible, reducing the number of animals used, minimising their suffering, and improving their welfare for as long as animal research continues.
Read the full statistics on animal use here.
Read the UK government’s strategy to phase out animals here.
*Data for 2014 and 2023 used in this post are corrected and revised. It is standard practice across all Home Office statistical releases to incorporate revisions to previous years’ data in the latest release. For more information, please visit the User guide to annual statistics of scientific procedures on living animals.
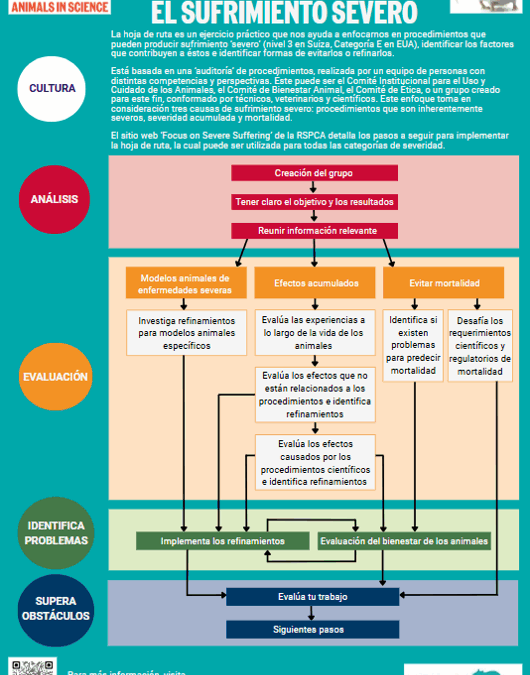
¡Buenas Noticias! The Roadmap resources are now available in Spanish
We are delighted to share a significant milestone in our ongoing commitment to ending severe suffering in lab animals globally: the Roadmap poster and the prospective procedures and lifetime experiences sheets, with their corresponding guidance notes, are now available in Spanish.
The Roadmap is a practical exercise to help you focus on procedures that could cause severe suffering, identify contributing factors and find ways of avoiding or refining these. However, the principles of the Roadmap can be applied to any level of suffering, not only severe.
By translating these materials, we are breaking down language barriers and making our resources accessible to a much broader audience, particularly in Spanish-speaking communities around the world. This initiative reflects our commitment to promoting widespread understanding of severe suffering and to encouraging best practices.
Resources in Spanish
- “Hoja 1 – experiencias de vida” (Sheet 1 – predicted lifetime experiences) helps you to focus on the harms of lifetime experiences of a lab animal. It can be used to discuss and predict what the animal could experience, what the welfare issues might be and how these could be mitigated. It is accompanied by a useful “Hoja 1 – ejemplo” (Sheet 1 example) to help you get started.
- “Hoja 2 – enfoque en procedimientos” (Sheet 2 – focus on procedures) helps you to focus on the harms of procedures. It can be used to identify what the animal is likely to experience during the procedures, as well as the welfare issues and ways to mitigate these. Relevant sections of this form are the steps within potentially severe protocols, methods of killing, animal experience and humane endpoints. This sheet also comes with “Hoja 2 – ejemplo” (Sheet 2 example) for guidance.
- In addition, the Roadmap poster is a convenient resource that can be printed out and displayed at your establishment, or shared with colleagues.
Other languages
The Roadmap poster is still available in English, French, and German. We are eager for more institutions to try out the Roadmap, and we would be happy to discuss its implementation, or conduct workshops in person or online.

New information sheet: Avoiding mortality in animal research and testing
We are pleased to announce the release of our latest information sheet: ‘Avoiding mortality in animal research and testing’. This information sheet specifically addresses unexpected mortality in animals, which is usually assumed to be preceded by ‘severe’ suffering unless there is evidence otherwise.
This free resource offers a framework that helps members of Animal Welfare and Ethical Review Bodies (AWERBs), and other similar committees, to proactively identify ways to avoid mortality within their establishments. It was designed to facilitate constructive dialogue and the implementation of effective strategies. The information sheet also includes a useful flowchart that outlines clear steps to help avoid mortality in animal research and testing.
You can download your free copy here.

New information sheet: Roadmap to reducing ‘severe’ suffering
We are pleased to share our new information sheet “Roadmap to reducing ‘severe’ suffering”. This resource has been developed to enable all members of Animal Welfare and Ethical Review Bodies (AWERBs) to help reduce, and avoid, ‘severe’ suffering at their establishments and to advise on refining procedures at all severity levels. It is also relevant to members of other similar bodies worldwide, such as Animal Welfare Bodies, Animal Ethics Committees and Institutional Animal Care and Use Committees.
The Roadmap is a free, user-friendly exercise that provides a structured and practical framework that enables users to:
- Evaluate procedures that could cause ‘severe’ suffering, focusing on the three main reasons for ‘severe’ suffering (i.e. severe disease models, cumulative effects and mortality), although it can be applied to any level of suffering.
- Identify contributing factors and implement refinements, using tailored welfare assessment protocols to ensure their effectiveness.
- Overcome any obstacles to refinement, review your work and plan the next steps.
The new sheet explains how to use the Roadmap and can be downloaded here. It is ideal for introducing the concept of the Roadmap to your establishment and encouraging people to trial the approach.
We have received extremely positive feedback about the Roadmap from many individuals and organisations, and we encourage users to download and use this resource to support their ongoing commitments to reducing suffering and improving animal welfare.

New report on refining bone marrow ablation and reconstitution
At the end of 2021 and beginning of 2022, we held two expert working group meetings on refining studies involving bone marrow ablation and reconstitution in mice. These procedures are used in different fields of research including studies of immune system function, ageing and cancer biology, and can induce ‘severe’ suffering in the animals used. The working group identified key welfare issues and set out practical refinements to reduce suffering and improve welfare. Our report, which is free to download and includes a flow chart to help you implement practical refinements at every stage, can be found here.

Meeting reports from Focus on Severe Suffering events 2024
We’re pleased to share that the reports from our two Focus on Severe Suffering events held in November 2024 are now live.
“Minimising Pain” at Newcastle University
The meeting featured two sessions: “Refining Pain in Pain Research”, focusing on reducing pain in studies where pain is the subject of research, and “Refining Pain in Painful Procedures”, addressing pain reduction in areas causing severe suffering without pain as the objective.
Read the full report here.
“Refinements of Animal Studies with a Focus on Severe Suffering” at Gentilly, France.
Our 6th international Focus on Severe Suffering meeting took place at Sanofi in Gentilly, organised in collaboration with FC3R, AFSTAL, GIRCOR, OPAL, Sanofi, RN-SBEA, RN-CEEA, and DCL Solutions. The one-and-a-half day meeting featured case studies of refinements, guidance on how to use the RSPCA Roadmap to reduce severe suffering, and the role of Animal Welfare Bodies (AWBs).
Read the full report here.
Both reports summarise the key presentations, discussion points, and actionable steps to help the research community refine practices and reduce suffering. We hope these insights enable further efforts to reduce and avoid severe suffering. Get in touch with any feedback, thoughts or suggestions at animalsinscience@rspca.org.uk.

Latest uk statistics on severe suffering
On 11 September, the 2023 statistics on animal research in the UK were released. We are encouraged by the continued decline in the number of animals experiencing ‘severe’ suffering. This represents significant progress since 2014 towards our goal of ending severe suffering in animal research.
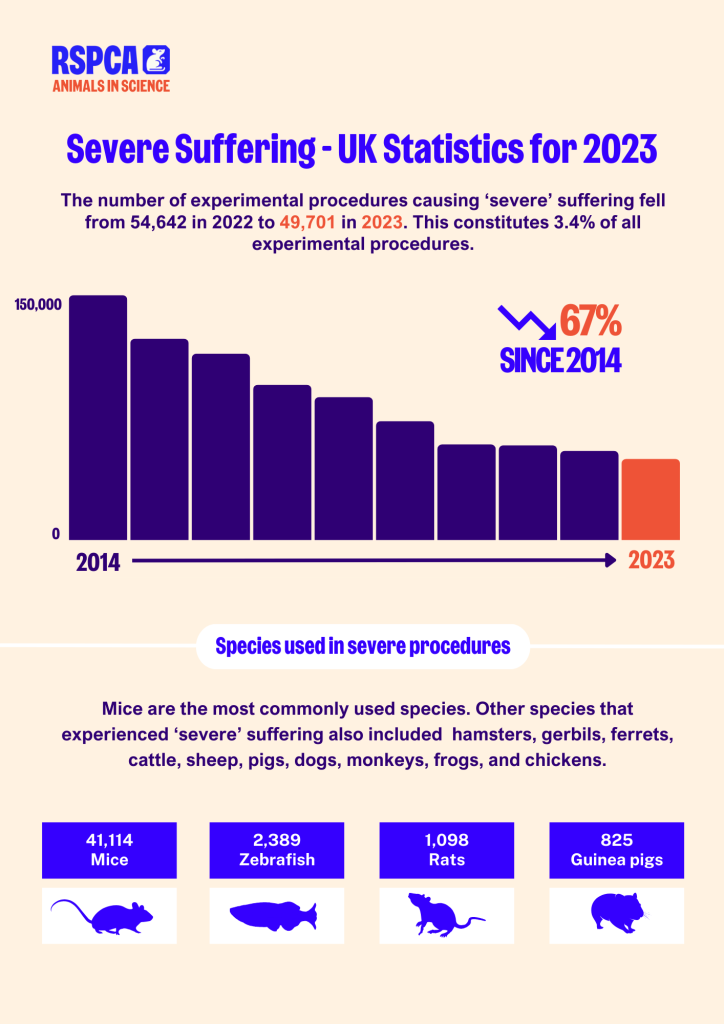
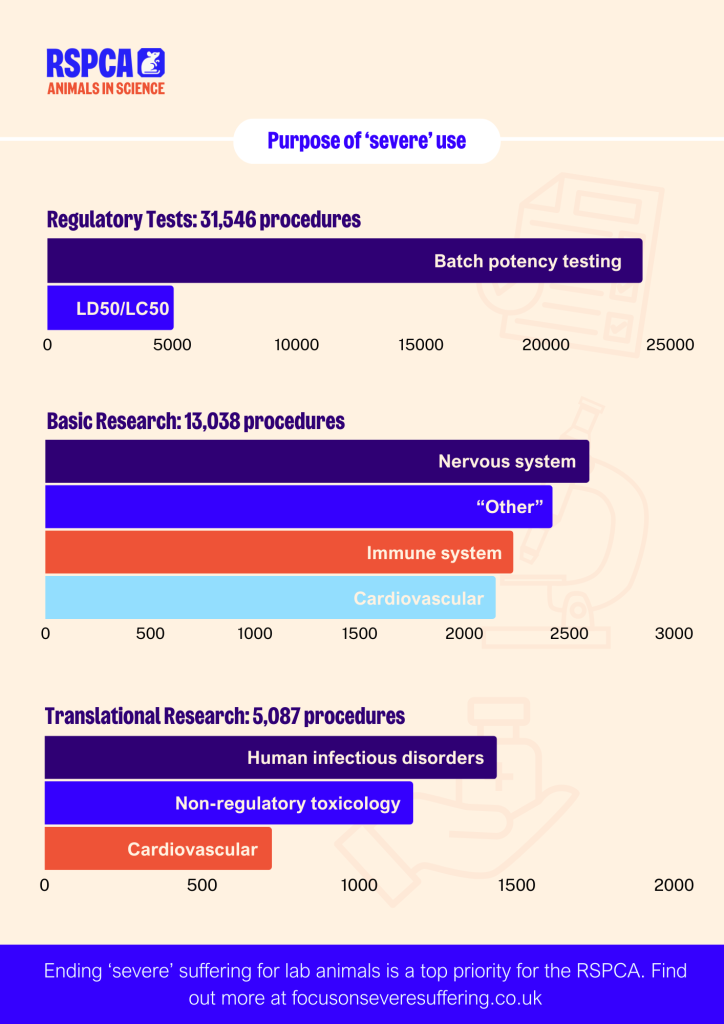
While ‘severe’ animal use in basic research increased slightly by 5%, regulatory use and applied/translational use decreased by 11.5% and 20.7% respectively. Mice remain the most commonly used species, accounting for 82.7% of all severe procedures. Batch potency testing and toxicity testing (LD50/LC50) account for 68% of all severe procedures involving mice.
The continuing reduction in severe procedures since 2014 reflects the efforts of the scientific community to minimise animal suffering. However, certain areas, such as batch potency testing, neuroscience, immune system and cardiovascular research, have proven more challenging to refine.
Our upcoming work will aim to address these crucial areas to advance the goals of our ‘Focus on Severe Suffering’ initiative.

The OBSERVE Guidelines
Cancer research represents one of the highest uses of animals across both basic and translational research. In basic research, oncology ranks as the third highest sub-category for causing ‘severe’ animal suffering, while in translational research, human cancers rank the second highest. Approximately 95% of this research is conducted using mice.
While guidelines like PREPARE and ARRIVE focus on planning, reporting, and ensuring reproducibility in all animal experiments, there has been a notable lack of standardised recommendations for the practical refinement of animal models in oncology. To address this gap, the Oncology Best-practices: Signs, Endpoints, and Refinements for in Vivo Experiments (OBSERVE) guidelines were developed.
The OBSERVE guidelines represent a consensus statement crafted by a select steering committee, led by Stéphanie De Vleeschauwer from KU Leuven, incorporating insights from experts across academia and industry.
In a stepwise approach, the OBSERVE guidelines aim to address multiple aspects of an in vivo cancer study, focusing on rodents:
- Preparation and Refinement of Implantation Methods: Ensuring the appropriate setup and refinement of specific implantation techniques.
- Assessment of Clinical Signs: Detailed descriptions of clinical signs associated with specific tumour types and guidance on how to assess these.
- Monitoring and Humane Endpoints: Guidelines for monitoring tumour growth, assessing animal welfare through standardised monitoring sheets, and applying humane endpoints.
The OBSERVE guidelines offer practical, clear, and robust recommendations for scientists, veterinarians, and animal care staff to reduce the levels of suffering experienced by animals during cancer research.
Read the OBSERVE guidelines in full here.
Latest statistics on ‘severe’ suffering in the EU for 2021 and 2022
On July 19, 2024, the European Commission released the most recent statistics on the use of animals in research and testing in the EU and Norway for the years 2021 and 2022.
These statistics play a vital role in the RSPCA’s ‘Focus on Severe Suffering’ initiative, providing crucial insights that can help target strategies to more effectively reduce severe suffering in animal research.
Overview
In 2021, there was a significant increase of 1.2 million in the total number of animal procedures. This rise was primarily attributed to three projects involving salmon and sea bass, which together accounted for 1.3 million animals. As a result, severe procedures also increased significantly that year.
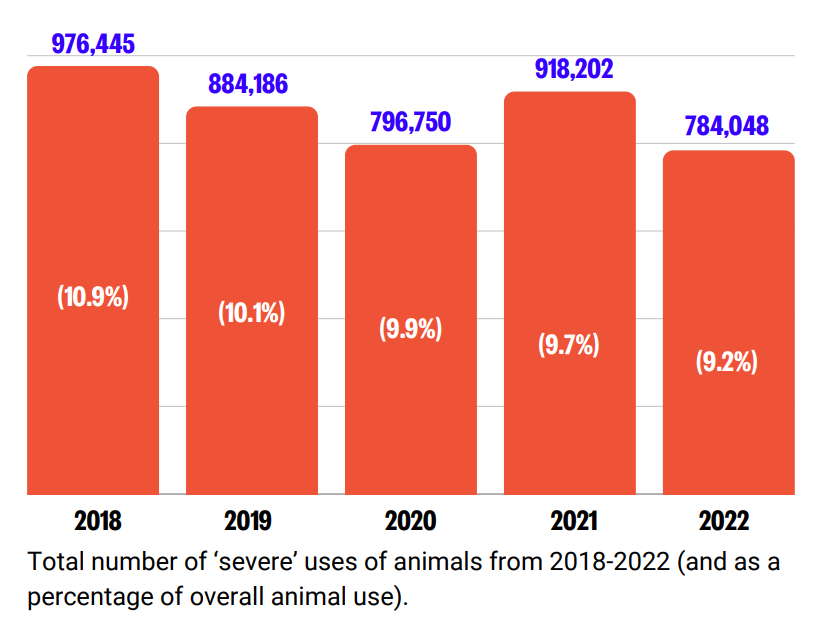
In 2022, overall animal use decreased by approximately 1 million. There was also a reduction in severe procedures.
Despite these fluctuations in the total number of procedures, the proportion of ‘severe’ procedures has continued a downward trend since 2018, dropping from 10.9% to 9.2% in 2022.
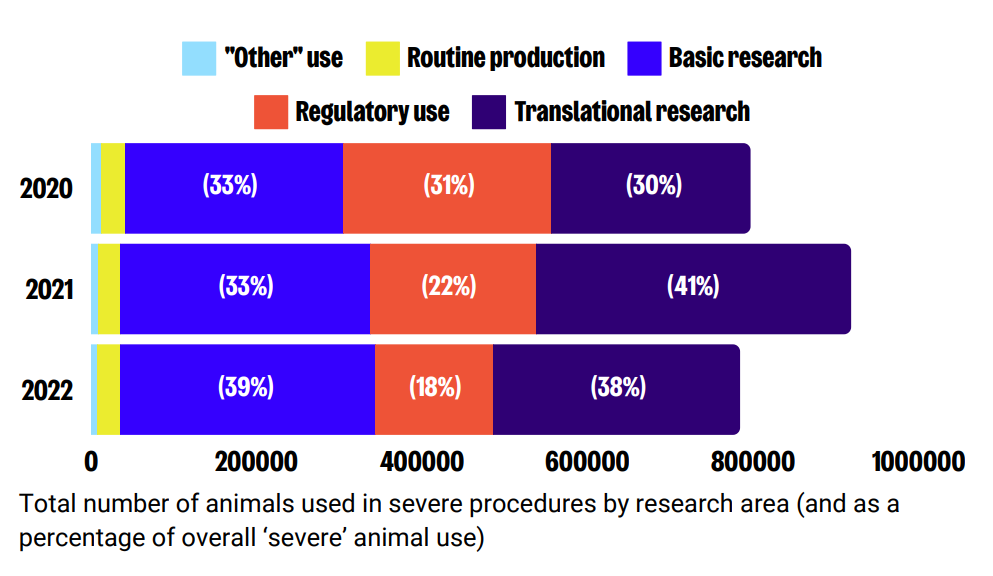
Main categories of ‘severe’ use
Over the last three years, basic research and translational research accounts almost equally for the highest proportions of ‘severe’ animal use. However, ‘severe’ regulatory use has decreased dramatically from 31% of overall severe use in 2022, to 18% in 2022. This decrease is attributed to continuous reductions in procedures related to medicinal products for human use.
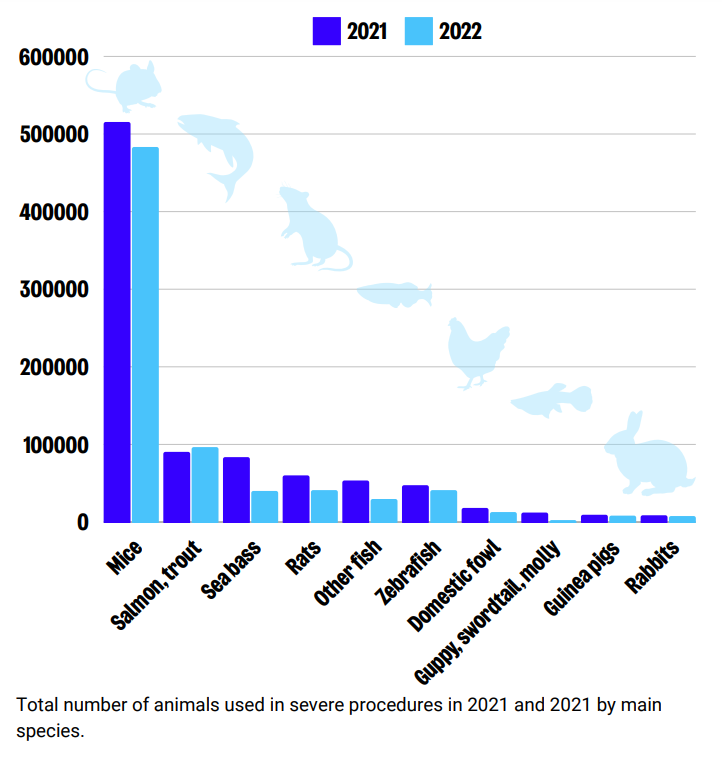
Species
Mice account for the highest number of animals experiencing severe suffering, followed by salmon, trout, sea bass and rats. Other species which experienced severe suffering, not shown on this graphic, include pigs, hamsters and sheep.
Sub-categories of ‘severe’ use: Basic research
In basic research, the greatest numbers of severe uses was reported for research on the nervous system, immune system, and oncology. Between 2021 and 2022, figures remained relatively stable across sub-categories of use.
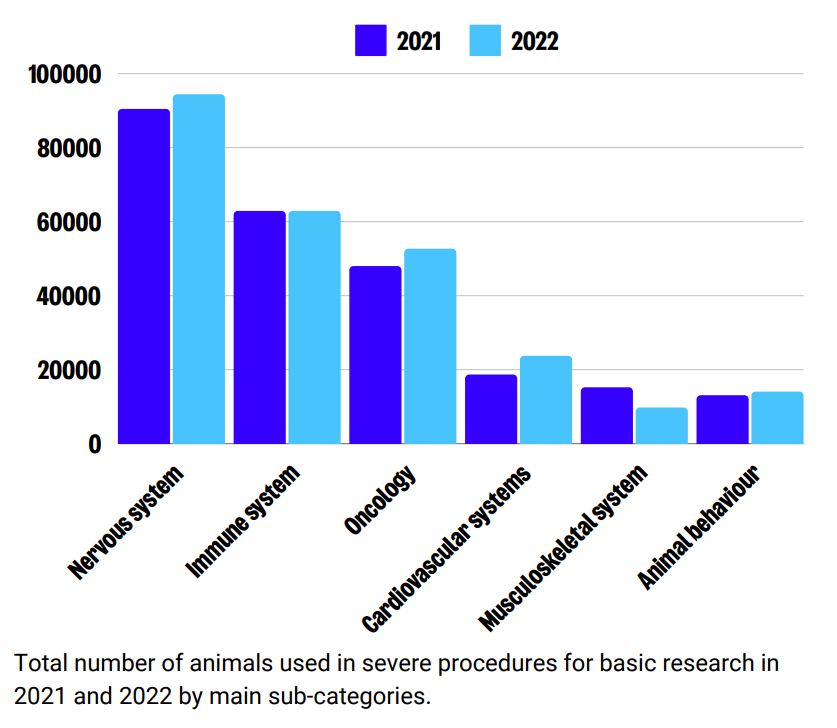
Sub-categories of ‘severe’ use: Translational and applied research
Animal diseases and disorders account for the highest number of severe uses across 2021 and 2022. Other figures remained stable across sub-categories of use in these years, with human cancer and human infectious disorders also resulting in high numbers of ‘severe’ animal use.
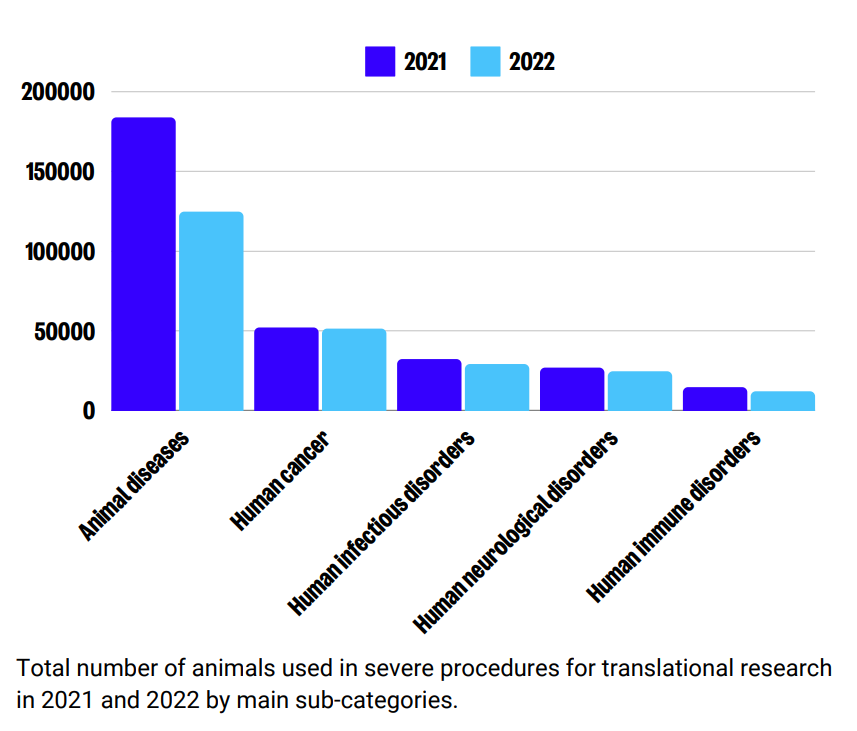
Sub-categories of ‘severe’ use: Regulatory use
‘Severe’ use of animals for regulatory purposes reduced by 20% in 2021, and 29% in 2022. This may be explained by efforts by companies and regulators to reduce and replace animal uses related to medicinal products. Since 2018, uses for legislation on medicinal products for human use has decreased by 50.2%. Better identification of clinical signs and application of earlier humane endpoints may also have contributed to this reduction.
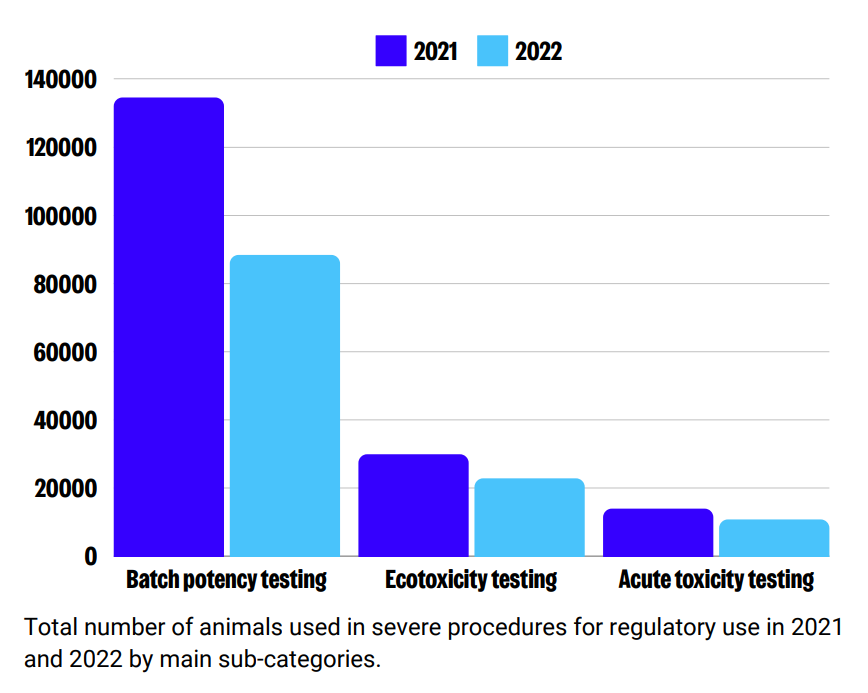
Sub-categories of ‘severe’ use: Routine production
The production of monoclonal antibodies by the mouse ascites method, which involves large, painful tumours, is responsible for the vast majority of severe uses in this category. 99.5% of all use occurs by one member state, France. There continues to be a lack of specific scientific, technical or other detail publicly available as to why use of this method continues to persist, despite all other Member States having transitioned to alternative approaches, and/or no longer permitting the use of the ascites method.
‘Severe’ use of animals for monoclonal antibody production by the ascites method increased by 34% from 2020 to 2021, and by 18% from 2021 to 2022. A total of 24,228 animals experienced severe suffering in 2022, accounting for 50% of all animals used in ascites
production.
These increases are reported as being generally associated with an increase in production of a test used in France for the clinical followup of patients with long COVID; and the production of control batches necessary to validate plans to transition to antibody production by non-animal methods.
Conclusions
While there have been fluctuations in the total number of animal procedures, and severe procedures, over the past two years, there is an evident overall trend towards decreasing severe suffering. The continued decline in the proportion of severe procedures since 2018 reflects ongoing efforts by the scientific community to minimise severe suffering, particularly in regulatory use. However, these statistics also identify some specific areas in which reducing and avoiding severe suffering has proven to be more difficult. These include research into animal diseases and disorders, ecotoxicology and studies on the nervous system. Addressing these
areas will be crucial for advancing the goals of the RSPCA’s ‘Focus on Severe Suffering’ initiative, and we will continue to work constructively with scientists, veterinarians, animal technologists,
regulators and animal ethics committees to help achieve further refinements, as part of our work to promote the fullest implementation of the 3Rs.
Read the full reports here.
Reducing severe animal suffering and improving human wellbeing
Caring, empathetic staff play an essential role in reducing lab animal suffering and improving welfare. However, the emotional toll on staff, including animal technologists, veterinarians and scientists, can be significant – especially when animals suffer severely. Megan LaFollette, from the US 3Rs Collaborative, found that higher severity protocols lead to higher “compassion fatigue”. This state of physical and emotional exhaustion is common among professionals caring for, or using, lab animals and can lead to symptoms like depression, anxiety and even chronic physical ailments.
The RSPCA’s “Focus on Severe Suffering” initiative offers guidance on reducing suffering to improve animal welfare, but as set out above this will also improve human wellbeing. This is critically important, because the goal should be to support, and retain, caring people who empathise well with animals. The “Roadmap” to reducing severe suffering offers guidance on identifying refinements for both procedural and non-procedural effects, applying the theory of marginal gains. This approach is based on many small refinements collectively leading to significant overall improvements, enhancing the welfare of both animals and those who care for them.
The 3Rs Collaborative resource lists several sources of compassion fatigue, providing a very helpful tool to check in on staff wellbeing and to address any factors that may be contributing to emotional burnout, such as severe protocols. This helps to uphold a culture of care, fostering positive outcomes for animals, those working with them and scientific outcomes.
Find more information on the RSPCA’s Roadmap to reduce and avoid severe suffering. If you would like us to visit your institution for a workshop to demonstrate the Roadmap in practice, contact us at animalsinscience@rspca.org.uk.

RSPCA’s 6th international Focus on Severe Suffering meeting
On 20-21 November 2024, the RSPCA will hold an international meeting in France on ‘refining animal research with a focus on severe suffering’. Building on the success of previous meetings held in Brussels, Berlin, Athens, Stockholm and Leiden, the meeting will be the 6th in a series of international meetings in our ‘Focus on Severe Suffering’ initiative.
Find the reports from previous meetings here.
The meeting will be held near Paris starting on 20 November from 9am, finishing on 21 November at 12 midday.
The RSPCA is partnering with other organisations that share the aim of reducing and avoiding severe suffering – Afstal, FC3Rs, Gircor, OPAL, RNSBEA, RNC2EA, Sanofi, and DCL solutions – to maximise the success and impact of the meeting.
The event will be open to all those directly involved in animal research, and will be free to attend. It will offer a unique opportunity for attendees to engage in insightful sessions featuring case studies, exchanges of best practice, and discussions on refinement strategies in a variety of research fields that have the potential to cause severe suffering.
Stay tuned for more information as preparations unfold. In the meantime, sign up to our newsletter to receive a notification when registration opens.

Reducing Severe Suffering using the 3Hs Approach
Reducing severe suffering in animal research involves a series of steps which are outlined in our ‘Roadmap’. A key objective is to identify and reduce ‘cumulative suffering’ by reviewing both procedure-related and non-procedure-related experiences throughout an animal’s life. While refining the scientific procedure is crucial, it’s important to recognise that the procedures often last a short time in comparison to the other lifetime events an animal may experience. Therefore, maximum consideration should be given to how animals are handled, housed, and habituated, to minimise severity as much as possible. This is especially important in severe procedures, which have the greatest impact on animals.
On March 27, a team of Neuroscientists at the University of Bristol, led by Professor Emma Robinson, launched the 3Hs Initiative. The concept of the 3Hs is to focus on refinements to rodent Handling, Housing, and Habituation, increasing animals’ positive experiences while reducing cumulative suffering.
Housing
Housing mice and rats in ‘standard’ laboratory cages contributes to cumulative suffering as animals are restricted in movement, choice of social partners, and a lack of stimulating or complex enrichment – which leads to negative welfare states. Practical solutions include providing playpens and sensory stimuli which can be maintained alongside standard caging, supporting positive welfare while also meeting the scientific objectives.
Handling
Physical restraint is widely used and considered necessary for both husbandry and scientific procedures. But restraint is aversive and causes distress, leading to negative associations between the human handler and animal. The 3Hs resource provides training on how to reduce and avoid restraining animals during common procedures, including techniques such as modified handling which minimises restraint during injections.
Habituation
Habituating an animal to a procedure can play a key role in minimising levels of suffering. If animals are gradually habituated to a procedure and develop a positive association to it, restraint may no longer be necessary, greatly improving the experience of the animal. The 3Hs resource provides detailed instructions on refined habituation protocols, such as positive reinforcement using food rewards.
You can read more about the 3Hs approach, and download protocols and SOPs, here.

Replacing botulinum toxin testing to reduce severe suffering
Botulinum toxin (Bt) is a neurotoxin which induces muscle paralysis by blocking neurotransmitters. Bt is used for treating medical conditions (such as migraines and lazy eye), and in cosmetic procedures to reduce the appearance of wrinkles.
The manufacture of Bt products is approved for medical purposes – and the use of animals in testing procedures is authorised for that reason. However, huge numbers of practitioners use them “off-label” for aesthetic treatments. “Off-label use” is known to be widespread, but it is not known what percentage is used for cosmetic versus medical applications. Indeed, there has been a documented surge in demand for Bt-based products within the cosmetic industry. In 2022, the International Society of Aesthetic Plastic Surgery estimated over 9 million “Botox” treatments were administered, a global increase of 26.1% compared to 7 million in 2021.
As Bt is a biological product, the potency of each batch needs to be tested and assured before it can be used in humans. The standard method, an LD50 assay using mice, determines the lethal dose that kills 50% of test animals. The increasing demand of Bt products has involved large numbers of animals undergoing ‘severe’ procedures.
In recent years, there has been substantial progress towards the replacement of Bt testing in animals. The manufacturer of Botox®, Allergan, was the first to develop its own cell-based potency assay in 2011, aimed at replacing the use of animals in batch potency testing for Botox. Allergan is based in Ireland, and a review of the available statistics from 2013-2022 reveals a marked decrease in the number of animals used in regulatory testing and undergoing severe procedures.
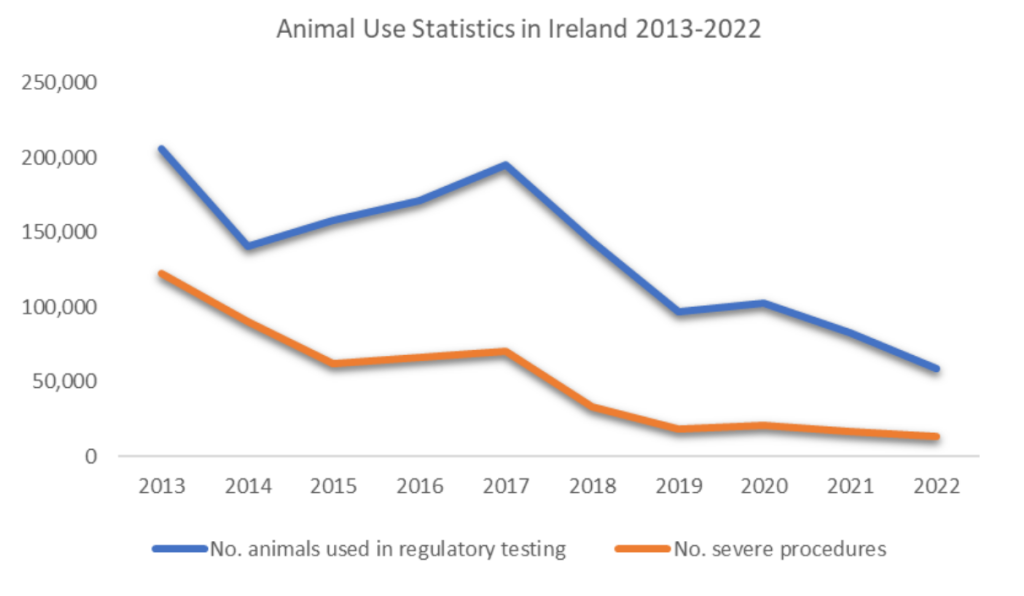
Between 2013 and 2022, there was an 89% decrease in the number of severe procedures in Ireland. The statistical reports do not provide enough detail to exclusively credit this decline to the replacement of batch potency testing of Bt. However, it is certainly feasible that the approval and use of the cell-based potency assay has played a significant role. If this is the case, transitioning from traditional animal testing methods to in-vitro alternatives substantially reduced severe suffering.
Unfortunately, the LD50 test continues to be used for batch potency testing of other versions of ‘Botox’ type products in Ireland and globally. Because each manufacturer produces Bt slightly differently, an alternative to the animal test needs to be validated for every company, and regulators in all jurisdictions where the product will be used need to accept these. Unfortunately, more animals will experience severe suffering until this is achieved. Also, manufacturers who are successfully using a cell-based alternative for batch potency tests may still use animals for other parts of the production process. The significant animal suffering still involved in the production of these products highlights the importance, and urgency, of enabling more rapid transitions to non-animal technologies and approaches.

2022 statistics on ‘severe’ suffering in canada
The Canadian Council on Animal Care (CCAC) publishes an annual report on the number of animals used in Canada for the purposes of research, teaching, and testing at CCAC member institutions. Canada employs a system of five ‘categories of invasiveness’ ranging from A to E, which roughly align with UK ‘severity classifications’. Category D describes ‘experiments which cause moderate to severe distress or discomfort’, while Category E describes ‘procedures which cause severe pain near, at, or above the pain tolerance threshold of unanesthetized conscious animals’.
On October 19, 2023, the CCAC released the 2022 data report on animal use of animals used in Canada. These statistics include data on severity, which is important for transparency and ethical considerations.2022
In 2022, 105,253 animals were used in the most severe category of protocols, Category E. This represented 2.8% of all animals used in research and testing in those Canadian institutions certified by CCAC.
What types of use were most likely to be severe?:
- 50.1% were used for regulatory testing to protect humans, animals, or the environment
- 30% were used for fundamental scientific research
- 11.8% were used for medical and veterinary studies related to human and animal diseases
- 7.8% were used for developing medical products
- 0.3% were used for educational purposes in post-secondary institutions
What animals were most often used in Category E studies?:
- 41,375 Rainbow trout
- 17,995 Mice
- 14,435 Salmon
- 14,267 Sticklebacks
- 5,690 Guinea pigs
- 2,478 Rats
- 3,660 Northern pikeminnows
This data report highlights the severe suffering experienced by fishes in fundamental research and regulatory testing. Notably, the data reveals that 37,027 rainbow trout were subjected to Category E procedures, specifically for regulatory testing purposes. This figure accounts for 35% of all Category E procedures reported by the CCAC. The report emphasises the urgent need for a critical evaluation of practices to minimise this suffering in regulatory testing. In line with this objective, we are organising a one-day workshop in Surrey dedicated to the application of humane endpoints in regulatory toxicology for fishes. Further information about this event can be found here.
The full CCAC data report and data files are available on the CCAC website.
2022 Statistics on ‘severe’ suffering in the UK
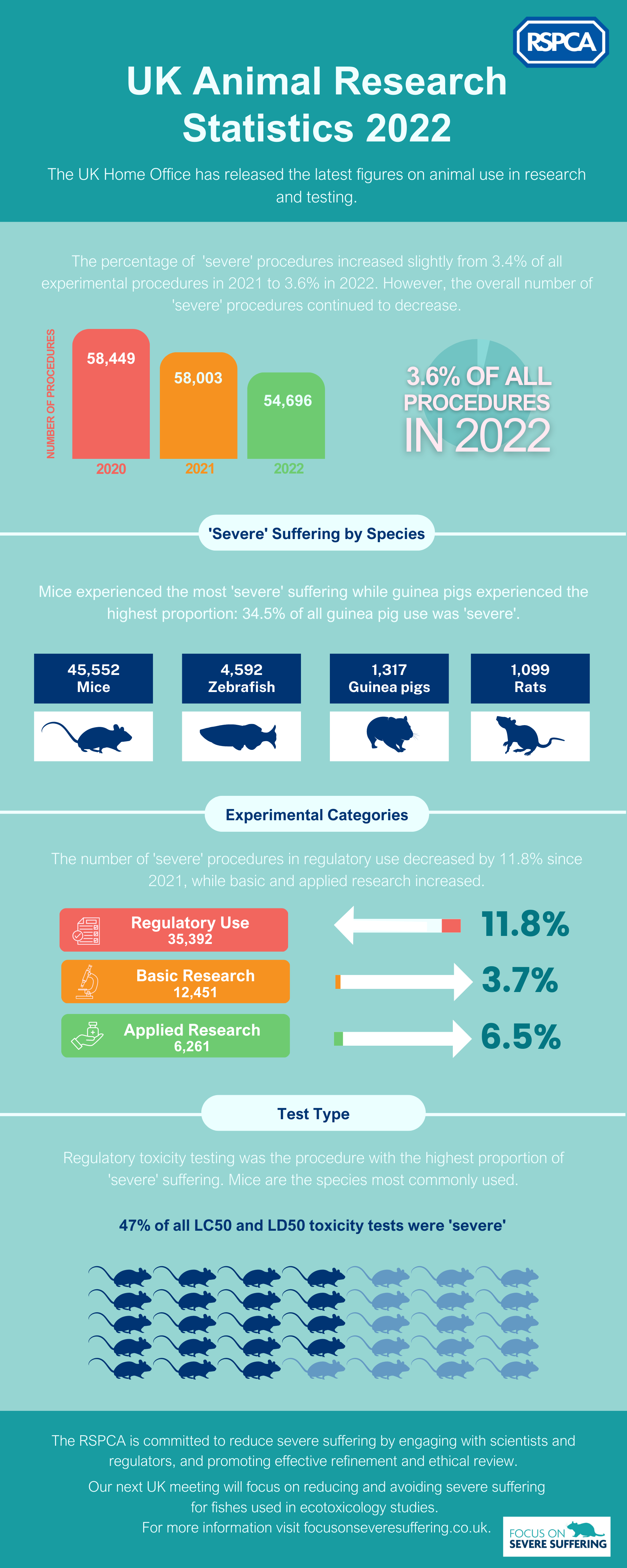
There has been a significant decline in the number of laboratory animals experiencing ‘severe’ suffering in the UK. Since 2014, there has been a remarkable 61% reduction in experimental procedures causing ‘severe’ suffering. The 2022 statistics continue to demonstrate this trend, although between 2021 and 2022 there was a slight increase in the proportion of severe procedures, from 3.4% to 3.6%.
Regulatory use accounts for the highest percentage of ‘severe’ suffering, and it is therefore encouraging to see that this has decreased by 11.8% since 2021. To further address this issue, our upcoming meeting in the UK, on 16th November, will focus on animal use in ecotoxicology. We will bring together scientists, animal technologists, veterinarians and trainers to discuss ways in which humane endpoints can be implemented more effectively. More information about this meeting will be posted on our events page, and registration details can be found here.
The full report can be found on gov.co.uk.

2020 Statistics on ‘severe’ suffering in the EU and Norway
On April 4, 2023, the European Commission released the latest statistics on the use of animals in research and testing in the EU and Norway in 2020. The UK is no longer included in the statistics since withdrawing from the EU, so the statistics from previous years have been recalculated.
This information is very important for promoting transparency and openness. It also plays a vital role in directing efforts more effectively towards the 3Rs in animal research. This summary of the data outlines the number of animals reported to have experienced ‘severe’ suffering.
Key findings
- Mice are the species most likely to experience severe suffering
- Batch potency testing is responsible for the largest number of severe procedures (134,017)
- Ecotoxicity testing has the highest proportion of severe procedures – 42% of all ecotoxicity testing procedures were severe
- The production of monoclonal antibodies by the ascites methods has increased by 12%. However, the number of severe procedures has decreased significantly
Overview
In the EU and Norway, 10% of procedures (796,750 in total) were severe, which is the same proportion of severe procedures as reported in 2019. The absolute number of severe procedures fell by almost 10%, from 884,186 in 2019, presumably because fewer animals were used overall due to the pandemic.
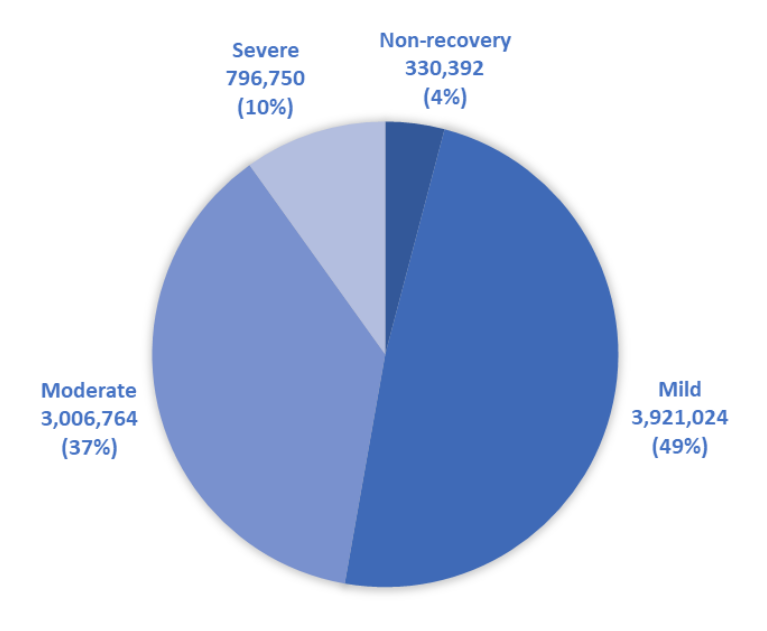
Which animals experienced the most ‘severe’ suffering?
- 492,464 Mice (12.5% of all mouse use was ‘severe’)
- 50,921 Rats
- 33,388 Zebrafish (11.9% of all zebrafish use)
- 166,855 Fishes (other than zebrafish)
- 13,638 Guinea pigs (12.2% of all guinea pig use)
Which types of use were most likely to be severe?
In 2020, the highest number of severe procedures were for regulatory purposes, translational and applied research, and basic research.
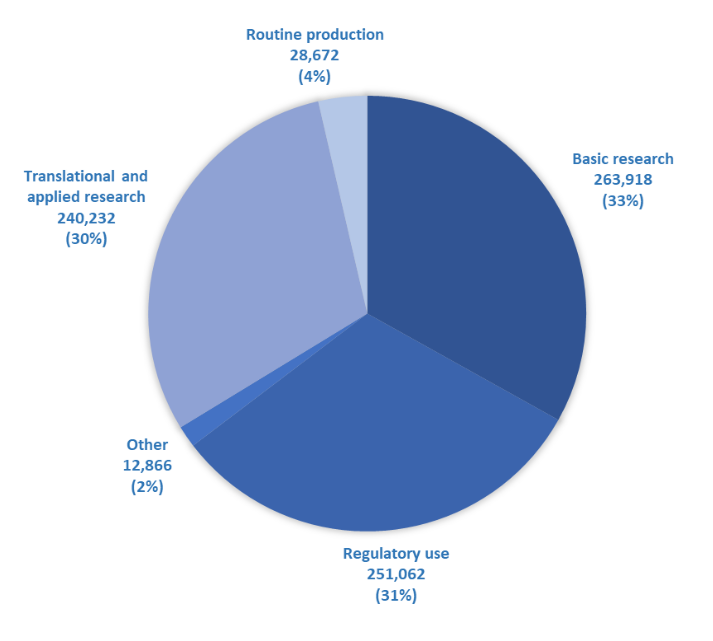
The graph below summarises which sub-categories of purposes have the highest number and proportion of severe procedures.
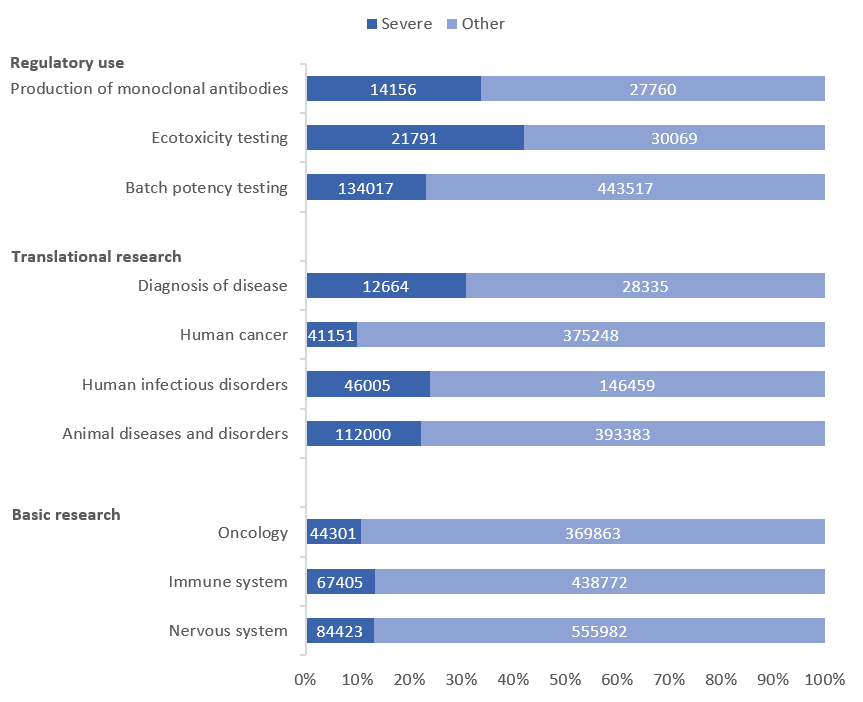
Batch potency testing had the highest overall number of severe procedures, followed by animal diseases and disorders, and studies on the nervous system. Encouragingly, there has been a 23% decrease in ‘severe’ actual severity associated with batch potency testing since 2019.
The use of the mouse ascites method for the production of monoclonal antibodies continues to be of concern. Alternative approaches are available and this method has not been used in the UK for example since 2012. Although use of this method decreased by 35% between 2018 and 2019, 2020’s figures saw it increase by 12%. This increase, and its use generally, was almost entirely reported by one member state – France. Improved application of refinement techniques and earlier endpoints are probably responsible for the reduction in the associated severity reported – 34% now being reported as severe, compared to over 90% in the past. The statistical report highlights that further efforts are apparently taking place to review ongoing projects involving the use of mouse ascites.
The full report on EU and Norway animal research statistics can be found on the ALURES database.
Roadmap now available in dutch
We are delighted to announce that our Roadmap to reduce severe suffering is now available in Dutch. Thank you to the National Animal Testing Policy Advisory Committee for providing the translations.
Our Roadmap is still available in English. We are eager for more institutions to try out the Roadmap, and we would be happy to discuss its implementation, or conduct workshops in person or online.

International Focus on Severe Suffering meeting, 12 to 13 October 2023
Our 5th RSPCA international meeting will include the usual popular combination of case studies and opportunities for discussion and networking, with a special focus on cumulative severity.
This 1.5 day, face to face meeting will be held in Leiden, The Netherlands, in association with the Netherlands National Committee for the Protection of Animals used for Scientific Purposes (NCad), Leiden University Medical Center, Dutch Association for Laboratory Animal Science (DALAS) and the University of Leiden. Please click here for further information, and we hope to see you there.
Roadmap poster now in French and German
We are delighted to announce that our Roadmap poster is now available in French and German. Thank you to the Swiss 3R Competence Centre for producing the translations, and for enabling us to showcase the Focus on Severe Suffering project at a joint workshop on Reducing Severity, held on 27 February. We are planning to run the workshop again, so please check our events page for further announcements. Our Roadmap poster is still available in English, so please share all three versions and let us know how you get on with implementing the Roadmap.
2023 – the year of the Roadmap?
The Focus on Severe Suffering initiative has had a successful year in 2022, with inspiring UK-based and international events, lots of positive support from the scientific community, and published statistics showing further decreases in procedures causing severe suffering in both the UK and European Union. This has been due to a number of factors, including refined humane endpoints, improved husbandry and support for animals, better communication between people with different roles (especially where animal technologists and care staff have been consulted) and more involvement from bodies such as the Animal Welfare and Ethical Review Body (AWERB), Animal Welfare Bodies (AWBs) and Institutional Animal Care and Use Committees (IACUCs).
We have also had positive feedback about the Roadmap approach to reviewing severe procedures. There is a flyer (PDF 638 KB) about the Roadmap (right), which you might like to print and display, or share with colleagues. We are keen for more establishments to trial the Roadmap – which can also be used to refine moderate or mild severity procedures – and would be happy to discuss its use, or run workshops to help trial it, in person or online. Please contact us using the form at the foot of this page if you would like to know more.

New data on ‘severe’ suffering in the EU and Norway
The most up-to-date data currently available on the use of animals in research and testing in the European Union (EU), and Norway, was published by the European Commission on 15 July 2022. This information is important for openness and transparency and can also help to focus 3Rs efforts more effectively. Below is a summary of the data, which is for 2019, setting out the number of animals reported to have experienced ‘severe’ suffering, and in which areas of science.
As in previous years, the mouse is the species most likely to experience ‘severe’ suffering, and batch potency testing of vaccines and other substances (such as botulinum toxin) is the category responsible for the largest proportion of ‘severe’ animal uses.
But on a positive note, the number of ‘severe’ animal uses fell by 65,661 in comparison with the 2018 data. This means that the percentage of ‘severe’ uses decreased from 10% to 9%, which is a small step in the right direction.
999,264 uses of animals (9% of the total) were reported as causing ‘severe’ pain, suffering, distress or lasting harm. This included:
- 344,582 in basic research (7.2% of all uses for basic research involved severe suffering)
- 310,367 in applied research (10.9% of all uses for applied research involved severe suffering)
- 291,166 for regulatory purposes (12.2% of all uses for ‘regulatory’ purposes involved severe suffering)
Main categories of use causing severe suffering
- 215,735 – Batch potency testing (represents 21.6% of all uses of animals that were ‘severe’)
- 132,695 – Studies of animal diseases and disorders (represents 13.3% of all uses of animals that were ‘severe’)
- 104,164 – Nervous system (represents 10.4% of all uses of animals that were ‘severe’)
- 77,654 – Immune system (represents 7.8% of all uses of animals that were ‘severe’)
- 52,574 – Oncology (represents 5.3% of all uses of animals that were ‘severe’)
Which types of use were most likely to be severe?
The production of monoclonal antibodies by the ascites method was the most likely to cause ‘severe’ suffering – 91% of all uses for monoclonal antibody production using this method were severe. The ascites method was still being used in six Member States; principally in France, which accounted for around 36,000 out of 37,477 uses in this category. Note – alternative approaches are available to replace the mouse ascites method, and this method has not been used in the UK since 2012.
Severe suffering was reported in 39% of animals used for acute toxicity studies in ecotoxicity, and in 29% of animals used in LD50 and LC50 tests for acute and sub-acute toxicity testing.
Which animals experienced the most ‘severe’ suffering?
- 647,525 – Mice (11.7% of all mouse use was severe)
- 201,100 – Fishes other than zebrafish (9.8% of all use of fishes was severe)
- 64,070 – Rats
- 25,094 – Zebrafish
- 18,273 – Domestic fowl
- 16,127 – Guinea pigs (12.9% of all guinea pig use was severe)
- 7,382 – Xenopus (27.8% of all Xenopus use was severe)
- 6,336 – Rabbits
5,253 uses of animals for the creation of new genetically altered lines (1% of uses in this category) and 50,018 uses of animals for the maintenance of established lines of genetically altered animals (7.06% of uses in this category) were categorised as ‘severe’.
If you’d like to look at the EU data in more detail, see the Commission’s ALURES database.
New data on ‘severe’ suffering in the UK
The most up-to-date data currently available on the use of animals in research and testing in the UK was published on 30 June 2022. A total of 3.06 million scientific procedures were completed on animals in 2021, which is an increase of 6% from the 2.88 million procedures in 2020. This is due to two national lockdowns during 2020, which affected activity at research establishments.
However, it was positive to see that the percentage of ‘severe’ procedures fell once again, from 4% of all experimental procedures in 2020 to 3.4% in 2021. Below is a summary of the data on the number of animals reported to have experienced ‘severe’ suffering, and in which areas of science.
As in previous years, more mice experience ‘severe’ pain, suffering, distress or lasting harm than any other species. The percentage of guinea pigs experiencing severe suffering is highest, because of the use of this species in quality control batch potency testing. Batch potency testing of vaccines and other substances (such as botulinum toxin) is the category responsible for the most uses of animals reported as ‘severe’.
58,003 experimental procedures using animals (3.4% of the total) were reported as causing ‘severe’ pain, suffering, distress or lasting harm
40,126 for regulatory purposes (11% of all use for regulatory purposes was ‘severe’)
12,003 in basic research
5,874 in applied research
Main categories of research and testing involving severe suffering
(data represent the number of ‘procedures’ undertaken that were reported as causing ‘severe’ suffering)
31,645 Batch potency testing
5,067 Acute and sub-toxicity tests
2,870 Immune system
2,613 Cardiovascular system
2,041 Human infectious disorders
Which animals experienced the most ‘severe’ suffering?
(data represent the number of ‘procedures’ undertaken involving those animals)
48,090 Mice (5.2 % of all mouse use was ‘severe’)
1,145 Rats
1,256 Guinea pigs (25.6% of all guinea pig use was ‘severe’)
6,318 Fish
In addition, 28,782 (2.2%) of the procedures involved in the creation of new lines, and maintenance of established lines, of genetically altered animals (not used in experimental procedures) were categorised as ‘severe’.
Source: Home Office Annual Statistics of Scientific Procedures on Living Animals, Great Britain, 2021 – published 30 June 2022

International meeting in Stockholm, 24 to 25 August
We are taking registrations for our free international meeting, on 24 and 25 August 2022, which we are jointly convening with the Karolinska Institutet, Stockholm. This face to face event will include case studies describing how severe suffering was reduced in basic and applied research, with a separate session on regulatory toxicology.
We will also explore how National Committees, ethics committees and Animal Welfare Bodies can contribute, especially within tasks relating to following the development and outcome of projects. The final session will discuss how industry bodies, accrediting organisations and other professional bodies can help to develop a strategic approach to reducing severe suffering.
With twenty presentations from motivational speakers, and plenty of opportunities for questions, discussion and networking, this event will inform and inspire you to address severe suffering at your establishment and within other bodies you may be involved with.
We are delighted to offer free registration, and refreshments, thanks to the generosity of the Karolinska Institutet and RSPCA donors.

Critical Incident Reporting
Not all severe suffering is predictable – sometimes it can occur due to an unplanned, critical incident. The Critical Incident Reporting System in Laboratory Animal Science (CIRS-LAS) has been set up to enable users to add unplanned events to a database (anonymously, if required). The aim is to achieve a constructive, open and transparent error culture in laboratory animal science.
The CIRS-LAS database is open to contributors worldwide, to enable mutual learning and to help prevent avoidable incidents from being repeated. It is searchable by different keywords including species, incident type and disease model. We think it looks useful to browse through and reflect on whether there is a risk of any of the incidents, and how this could be reduced. Many would also be useful examples within education and training. It is easy to report incidents, and of course the more entries there are, the more useful the database will be.
CIRS-LAS is available in English, French and German at www.CIRS-LAS.de
For further information, please contact: info@cirs-las.de
AVOIDING AND REDUCING SEVERE SUFFERING
Avoiding and reducing severe suffering helps to fulfil legal requirements, reduce ethical concerns and improve scientific quality – this website will help you to achieve this.
Practical ways to reduce or avoid severe suffering include: