Roadmap steps
The best starting point is a collective agreement within an institution that ending severe suffering is desirable, possible and deserving of the necessary time and resources. This can be viewed as part of the local ‘Culture of Care’, which should include a shared responsibility and accountability for animal welfare and a positive collaborative relationship between animal technologists, veterinarians and scientists. Local committees such as the AWERB, AWB, IACUC or AEC play a key role in helping to promote a Culture of Care and could initiate the implementation of the Roadmap.
Depending on your individual facility, it may be useful to apply a ‘stretch objective’ to ending severe suffering, imposing a challenging but achievable time point after which no further severe studies would be undertaken. The target will obviously vary depending on the model or procedure being used and the obstacles identified. Having a fixed point in time to work towards can be a powerful motivating force for achieving challenging goals.
Implementing the Roadmap
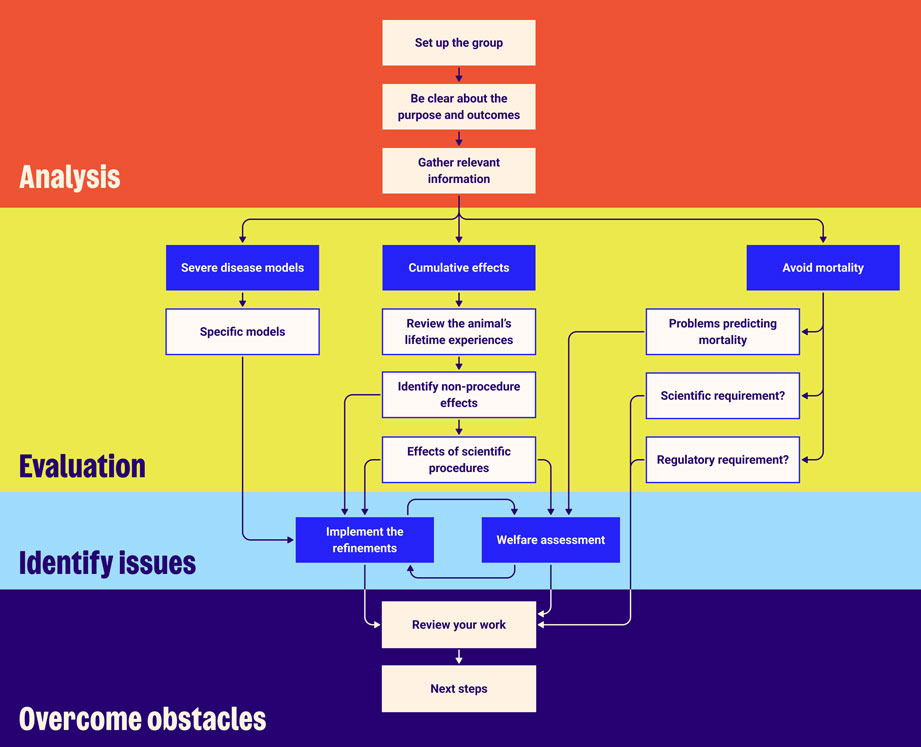
We have set out a step-by-step process (below) to help your team analyse and evaluate potentially severe protocols, identify any obstacles and set out ways to overcome these. You might like to suggest your institution gives it a try – and do please send us any feedback you may have: animalsinscience@rspca.org.uk
Sheet 1 - Predicted lifetime experiences resources
Sheet 2 - Focus on procedures resources
Other guidance
Step-by-step guide to carrying out the Roadmap exercise
Step 1
Analysis
-
This activity could be carried out by your institution’s Animal Welfare and Ethical Review Body (AWERB) in the UK (or the Animal Welfare Body, AWB, if in the EU, or similar body elsewhere), a 3Rs sub-group, or a specific committee set up for the purpose. You may wish to include the scientist undertaking the project, the veterinarian, animal technologists and ‘named’ persons, a lay or independent member, and a representative of the competent authority (e.g. local Home Office Inspector in the UK) if appropriate. You could also consider external expertise, for example on animal welfare science, each of the 3Rs or scientific knowledge.
-
The objective is to focus on procedures that could cause ‘severe’ suffering, identify contributing factors and find ways of avoiding or refining these.
Potential outcomes could be a record of key points from the discussion, with clear actions for specific individuals – information about which could also be shared with others (e.g. AWERB/AWB, Home Office Inspector) as appropriate.
Editorial changes to the protocols and steps detailed in the project application form may not be necessary, but it might be appropriate to include some outcomes within the written application.
The most important outcome is reductions in severity, with positive welfare impacts for animals, so good records and follow-up are essential.
-
You will need:
- A description of the proposed protocol, study or project
- The European Commission document on the severity assessment framework and its accompanying document with worked examples
- The Roadmap prospective procedures and lifetime experiences sheets, with guidance notes
Step 2
Evaluation
Severe disease models
-
Although many factors can combine to increase the risk of severe suffering, they can be divided into three main areas:
- some protocols are severe in themselves;
- some may include a number of steps that are not severe in isolation, but combine to cause severe suffering; so-called ‘cumulative severity’; or
- there may be a risk of animals dying (mortality).
If animals are ‘found dead’, severity is assumed to be severe unless there is evidence otherwise. -
Some protocols include models of diseases such as rheumatoid arthritis, sepsis, ALS or pancreatitis that may be severe by their very nature. There may be literature on refining the specific procedure in question; search for [disease] [animal model], [species] and [welfare] or [refinement] and also see RSPCA, 3Rs platforms,such as the UK NC3Rs and databases listed on the European Commission website.
Other sources of information include other scientists and researchers, animal technologists and veterinarians with experience in the field, the local Home Office Inspector (if in the UK), and relevant societies, organisations and user groups. Online discussion fora include COMPMED and VOLE, which require membership but colleagues should be able to access these.
Action points
Draw up plan (with deadlines) to retrieve further information about refining the protocol, tasking individuals with:
(i) searching the scientific and other relevant literature,
(ii) consulting internal and external colleagues and
(iii) posting questions on online discussion fora.
Cumulative effects
-
Animals experience many events during their lifetime, including scientific procedures and their impacts, plus transport, marking for identification, capture, handling, restraint, laboratory housing and husbandry, and humane killing. Some experiences may be painful, distressing, or may influence the animal’s ability to cope with regulated procedures – and the scientific outcome.
It is essential to consider how the effects of these experiences may interact; for example:
- Animals may become sensitised to certain procedures (e.g. repeated injections), so the suffering associated with each one is increased
- If recovery time is not sufficient following stressful events – such as cage change – before a procedure, the severity of the procedure may increase
- The impact of some procedures (e.g. surgery without the most effective perioperative analgesia regime) may be long-lasting or permanent
- Or, animals may habituate (become used) to repeated procedures, which can reduce suffering, especially if they can be trained using positive reinforcement techniques to avoid restraint
It is critically important not to make subjective assumptions about cumulative effects either increasing or decreasing – expert input and monitoring systems are needed to ensure that welfare issues, and refinements, are detected. -
Two essentials for understanding, assessing and reducing cumulative severity are (i) thorough review of the animal’s lifetime experiences, identifying every source of potential suffering and implementing refinement for each one, and (ii) an effective welfare assessment system.
The lifetime experience review should include:
- adverse effects directly caused by scientific procedures and their impacts.
- non-procedure related effects, e.g. stress due to cage change, which can also affect welfare.
-
Please use sheet 1, ‘Predicted lifetime experiences (Word)’ (you may wish to edit the first column) , the guidance notes and the worked example. See the lifetime experiences page for further guidance. You may wish to edit the first column.
You can use the sheet to discuss and predict what the animal could experience, what the welfare issues might be and how these could be mitigated (see example below). Many of these will already have been addressed in institutions and establishments that follow good practice, but it is a useful exercise to review them regularly in the light of new information about animal behaviour, welfare or refinement.
Input from named persons such as the veterinarian, should be especially valuable here. Following discussion, an action plan should be drawn up if there are any tasks that arise; for example, looking into new sources of animals or trialling different group sizes.
Further reading: the UK national Animals in Science Committee review of harm-benefit analysis includes a section on integrating cumulative effects.
-
We can never know exactly what an animal is experiencing, but knowledge about animal biology, behaviour and welfare can predict what an animal is likely to find painful, distressing or anxiety-inducing.
For example, an apparently simple procedure such as ear biopsy may seem trivial from a human perspective, but will be more significant from a mouse’s viewpoint. It involves capture and restraint, which are potentially distressing (especially if caught by the tail). A needle punch through the ear will be painful, and although acute pain is transient there may well be longer lasting effects of discomfort or pain and distress, requiring time to recover.
Although ear punching is likely to be preferable to tail tipping for biopsy, especially if combined with identification, the key message is the need to empathise and think of the animal’s experience step by step, identifying ways to reduce suffering. In the case of ear biopsy, this could include refining capture, providing a refuge to retreat to post-procedure and ensuring that staff are trained, competent and empathetic.
-
Please use sheet 2, ‘Focus on procedures (Word)’, with its worked example, and the project proposal or protocol description. Sheet 2 is based on the European Commission document with examples to illustrate the process of severity classification.
This stage involves looking at the project proposal or description of the protocol, reflecting on what will be done to the animal and what they are likely to experience. Relevant sections of the application form are the steps within potentially severe protocols, methods of killing, animal experience and humane endpoints.
Three key questions are:
- What is the source, nature and likely duration of suffering?
- What indicators can be used to identify any suffering?
- How will the suffering be avoided or reduced to a minimum?
In the UK, researchers are asked to summarise the typical experience or end-to-end scenario for an animal being used in each protocol, considering the cumulative effect of any combinations of procedures. For each step, the application should set out:- Expected adverse effects, including the likely incidence, and the anticipated degree and duration of suffering
- How each adverse effect will be monitored for, controlled and limited
- Humane endpoints
These should provide information that will help to fill out sheet 2, and/or identify further areas for discussion between the applicant, named persons such as the veterinarian and other members of the group. Go to Implement the refinements
Avoiding mortality
-
Directive 2010/63/EU, and the UK Animals (Scientific Procedures) Act 1986 (ASPA), state that death as an endpoint should be avoided wherever possible and that humane endpoints should be used instead. During the project evaluation process, any potential or requirement for death must be clearly identified and steps implemented to avoid this or evidence produced to justify it.
Three key questions are:
- Is there a scientific requirement for death as an endpoint?
- Is there a regulatory requirement for mortality?
- Is mortality difficult to predict in the strain or model?
-
If there is a proposed scientific justification for death as an endpoint, the applicant should be able to explain and defend this.
Action points
One approach could be to ask the applicant what they would do if they were simply told ‘no’ and had to implement humane endpoints instead? Would this have a negative effect on translatability? Could the experimental approach or design be altered to avoid mortality and still yield useful information? Could a pilot study be conducted to evaluate these questions? Go to Review your work -
Any perceived or actual regulatory requirements for death as an endpoint should be rigorously examined and critically challenged. For example, the OECD recognises that ‘with increasing knowledge and experience, investigators … will be able to identify more specific, early humane endpoints in the form of clinical signs for impending death or severe pain and distress. This would permit international harmonisation of these humane endpoints.’ Researchers and establishments should challenge regulatory bodies to accept evidence that death can be predicted and accept data obtained from tests with humane endpoints.
Action points
If it is believed that there is a regulatory requirement for death as an endpoint, task an individual or small group with researching this, to see how flexible the requirements are in practice and whether there is actually scope to implement humane endpoints. Go to Review your work -
For the purposes of actual severity reporting, the death of an animal must be reported as severe suffering unless an ‘informed decision’ can be made otherwise.
Some mortality is genuinely unpredictable or difficult to avoid – but knowledge and approaches to detecting relevant indicators are developing and there may be ways of reducing deaths or avoiding death completely.
Causes of mortality include:
- Background causes, unrelated to procedures
- Procedural causes
- Use of a high-mortality strain
Action points
It is important to challenge perceptions about inherent levels of background mortality. For example a 0.5 % incidence may appear to be low, but this could result in 5 deaths in every 1,000 animals. If relevant, the group could discuss what level of background mortality, if any, is ‘acceptable’, according to local values – and question the necessity and/or justification for using strains with a mortality rate above this. Would it be possible to answer the question using another strain?If the mortality is due to procedures and is difficult to predict, someone with appropriate expertise (e.g. the scientist or a named person) could be tasked to research and consult as to whether there is any new information on ways of predicting mortality within the protocol or strain. For example, telemetered body temperature using microchips has greatly improved the ability to predict death in a number of fields, such as vaccine testing. Go to Welfare assessment
Step 3
Identify issues
-
Ideally, you should now have a list of potential refinements to implement, which have been discussed with the researcher, animal care staff and relevant others, and trialled first if necessary. Some may lead to obvious benefits (e.g. soft, non-tangling nesting material for animals with arthritis), and others may require more structured evaluation. You will need to ensure that the welfare assessment protocol will also assess how effective any refinements have been. This will help to implement refinement in future projects.
-
Indicators of pain, suffering or distress (or positive welfare states) should be:
- Readily and reliably recognisable
- Effective at providing robust information about the animal’s welfare state
- Relevant to the study, species and strain
- Practical to carry out, without overly disturbing the animal
- Possible to consistently measure, interpret and analyse
It is important to set out a tailored welfare assessment, recording and monitoring system, or to refine the current approach. You can use the European Commission guidance on severity assessment as a template, with the help of your filled-in sheets from the Cumulative effects section, if you worked through this.The EC document on a severity assessment framework (p. 13 onwards) divides potential indicators into:
- Six high level categories: Appearance, Body Functions, Environment, Behaviours, Procedure-specific Indicators, Free observations; which are subdivided into
- ‘Areas to focus on’ e.g. coat and skin condition, enclosure environment, social interaction; and
- ‘Specific indicators to monitor’ e.g. lack of grooming, whether using enrichment, temperament change
Sheet 2 can be used to identify indicators to monitor, and using the EC document will help ensure you have an appropriate range of different indicators, including important high level categories.The welfare assessment protocol can be refined either at the time of review, or subsequently by a different group if there is one, e.g. the welfare assessment ‘team’ (see JWGR report on welfare assessment).
Step 4
Overcome obstacles
-
Your group may have covered some or all of these areas:
- Set out a plan to retrieve information on refining inherently severe protocols
- Reviewed lifetime experiences and the potential impact on the animals
- Worked through the protocol sheet
- Identified likely adverse effects, possible refinements, humane endpoints, welfare indicators
- Set out a plan to review and optimise the welfare assessment and recording system
- Planned to act on mortality
- Exploring the scientific justification
- Planning to review regulatory requirements
- Planning to find better ways to predict death
- Decided on outputs, e.g. reports, edits to the project proposal form
-
While everyone is still present, it is helpful to check the following:
- How will the outcome of the exercise be captured and communicated to all relevant people?
- Have any edits to the project proposal form or SOPs been recorded?
- Has at least one person, or group, been identified as responsible for each action?
- Are follow up meetings in the diary?